In the second part of our two-part interview with Daniel Goldstein, MD, an oncologist who studies the influence of business interests on healthcare, we talk about the capture of the health system by pharmaceutical companies.
In the previous installment of our two-part interview with Daniel Goldstein, we talked about capitalism and healthcare, what motivates physicians, and the effectiveness of the healthcare systems in the U.S., U.K., and Israel. In this part, we focus on capture: the influence that pharmaceutical companies and other health suppliers have over all aspects of the health system.
Guy Rolnik: Let’s go back to your notion that the pharmaceutical industry influences how medicine is being researched and provided. Would you say doctors and physicians are influenced by interest groups and drug companies and trade groups? And if yes, how?
Daniel Goldstein: For sure, it’s happening. If I take my field of oncology specifically, the pharmaceutical industry has a large amount of control. I wouldn’t say controls completely, but has a large influence over academic work, medical care, prescribing habits of physicians, and so on.
How does this happen? There’s an interplay in many, many different ways. Drug trials were once run by independent, government-funded cooperative groups that were independent of pharmaceutical funding. Most of that funding is disappearing, or [there are] very small amounts of funding.
GR: Because?
DG: Because the U.S. government is not committing large enough amounts of money.

GR: What you’re saying is that, in the past, a large chunk of the funding for these independent trials came from the government.
DG: Yes.
GR: And now the governments’ share is much smaller. Who makes up all the difference?
DG: Most new trials are what we call industry-sponsored trials, where the pharmaceutical industry develops the design of the trial and provides the funding.
GR: So, you are saying here that it’s not only the funding but also the actual design of the trials.
DG: Yes, they design the trials. Some of these trials may be skewed to the business interests of the company.
GR: Can you know they are skewed even before they start?
DG: Yes.
GR: What happens if you object to that? You’re a physician, you object to the design and say, “This and this is skewed.”
DG: That’s a good question. Many of the academic researchers, the big academic centers, receive much of their funding from the pharmaceutical companies to run their clinical trials. If they start putting up objections, saying for example, “No, we should use this dose and not that dose,” they might run the risk of being defunded or not having that trial opened at their center.
GR: The drug companies provide the funding and they design the trials. What entities actually perform the trials? Is it public entities, academic entities, or business entities?
DG: The trials are run out of academic hospitals. Basically, what we call a clinical trialist—who’s an academic physician—sees patients, recruits them, and puts them on the trial. It’s basically different hospitals. The trials are run through different hospitals. The hospitals get funding through that mechanism.
GR: So, it’s not just a question of funding by private entities but also the design of the trials.
DG: Yes. It’s problematic because they design the trial.
GR: Have you witnessed some of these trials?
DG: Yes. I put patients on clinical trials that we have running.
GR: And you’re aware of the design of the trial.
DG: Right. But the trial is given to us.
GR: But you were obviously aware of the design.
DG: Right.
GR: How many such trials have you taken part in?
DG: I’m young in my career. So far, I’ve recruited patients on maybe four or five trials.
GR: In Israel, right?
DG: Yes, but it’s the same in other places. You might have the same trial running in America.
GR: So, you personally took part in four or five trials. Did you have any cases where you thought that the design provided by the drug company was skewed, biased, or had no, or little, scientific value?
DG: Yes. There are trials where, scientifically speaking, they are not going to definitively answer the question the trial sets out to answer, or trials that are designed with a dose of a drug that’s too high and not as necessary. But don’t forget, this could also happen in a non-industry funded trial—perhaps it’s just part of the scientific process. But when there is also a business interest, it raises concerns that some of the decisions made in the trial design are business decisions and not scientific or medical decisions.
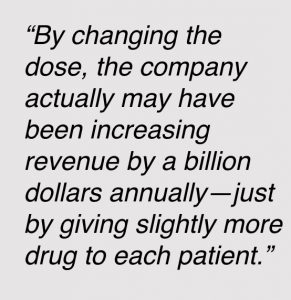 This relates to something we published a few weeks ago. It was when we released data as the company changed the dose of the drug from 2mg/kg to 200mg for all patients. They used that dose in a trial and that led to the FDA approval. By changing the dose, the company actually may have been increasing revenue by a billion dollars annually—just by giving slightly more drug to each patient.
This relates to something we published a few weeks ago. It was when we released data as the company changed the dose of the drug from 2mg/kg to 200mg for all patients. They used that dose in a trial and that led to the FDA approval. By changing the dose, the company actually may have been increasing revenue by a billion dollars annually—just by giving slightly more drug to each patient.
GR: Would it be correct to say that it is not uncommon to find a drug company-funded clinical trial that is skewed?
DG: That’s correct.
GR: Would it also be correct to say that it is skewed in a direction that would benefit the drug company?
DG: That’s also correct. Sometimes, one may argue it’s not intentional, but it may be intentional also.
GR: We talked about trials. Have you seen cases where drug companies influence academic basic science, and even not basic science? Do they have an influence over the projects that are chosen and those that are rejected?
DG: Let’s talk about journals and conferences, because those are two major arenas of academic prestige.
 Let’s first talk about journals. What’s interesting about journals is that the big academic journals, such as the New England Journal of Medicine (NEJM), or in my field, Lancet Oncology, often publish big trials run by the pharmaceutical industry that show a survival benefit of two months for patients. No cure, just increasing survival by two months. To me, many of these have minor clinical relevance to the majority of patients. It’s an improvement. Two months is not nothing but it’s not a massive survival benefit. The question you have to ask is, why are these journals— the most prestigious journals—publishing these trials, trials that show such a minimal clinical benefit? The answer may lie in the question of what’s called reprints.
Let’s first talk about journals. What’s interesting about journals is that the big academic journals, such as the New England Journal of Medicine (NEJM), or in my field, Lancet Oncology, often publish big trials run by the pharmaceutical industry that show a survival benefit of two months for patients. No cure, just increasing survival by two months. To me, many of these have minor clinical relevance to the majority of patients. It’s an improvement. Two months is not nothing but it’s not a massive survival benefit. The question you have to ask is, why are these journals— the most prestigious journals—publishing these trials, trials that show such a minimal clinical benefit? The answer may lie in the question of what’s called reprints.
If, say, I publish my own unfunded paper in a journal, I may get an email asking me to purchase “reprints”—glossy individual pamphlets of the paper. There is a lot of money in it: the original publisher is paid for reprints and pharmaceutical companies do purchase these reprints, perhaps in large numbers. It’s an undisclosed and undiscussed problem. It’s not clear how much journals are receiving in reprints from the pharmaceutical companies. It seems that that’s the mechanism, meaning that most of these industry-funded trials, which clinically offer no major breakthroughs, are being published in these prestigious journals. Sometimes they’re “me-too” drugs. Why? Because they’re getting money from reprints.
GR: Does it go as far as papers on drugs that offer only minor benefits are chosen over papers about trials on drugs that are probably more beneficial?
DG: I’m not saying drugs that have more benefit health-wise are rejected. Not necessarily. I just often wonder how journals make such editorial decisions. Are the decisions completely free from business interests? Maybe not? But it’s important for the company to publish in a prestigious journal, as it increases credibility for the treatment and may lead to increased sales.
GR: If money is involved, as you’re implying, it may mean that it has an editorial implication that distorts the picture compared with the situation where these incentives would not be present, right?
DG: Right. And there’s also the issue of conferences—the other major area of academic prestige.
A lot of academic activities are funded by the pharmaceutical industry. There’s been a lot of debate about how much should be funded and how much shouldn’t be funded, but arriving at that balance is tricky. If we take no funding from them, then we’ll essentially be having a small conference in a park, drinking water without any transport, and it would be difficult to continue academic activity.
GR: There may be other funding options, maybe the government or the academic institutions.
DG: Correct. Then the flip side is that the pharmaceutical companies are providing the vast bulk of financial support for conferences. Sometimes there is a wall between the funders and the conference organizers.
GR: Who is maintaining this wall? Editorial boards? Who?
DG: The conference organizers. However, obviously, if one particular conference is known for having a particularly large amount of anti-pharmaceutical industry material, it might affect their subsequent funding. Sometimes there’s no wall between the funders and the organizers and sometimes, even if there’s a wall, it’s not a complete, full wall. It’s perforated, which does affect how freely and openly individuals can speak at such conferences. Essentially at all academic meetings.
GR: Let’s try to understand how prevalent this is. Out of all the oncology conferences that you are aware of, how many are funded by drug companies?
DG: A hundred percent, which is problematic.
GR: At least in some cases, the amount and composition of funding is regulated. Aren’t there ways around these regulations?
DG: Yes. A drug company may be forbidden from paying for airfare, but it is still paying for it since participants may use research grants to pay for these flights—grants that are provided by drug companies.
GR: So, what the drug companies actually did, and correct me if I’m wrong, is take control of the entire funding cycle.
DG: Right.
GR: That means that, if they cannot fund you directly as sponsors of the conference, they can fund you indirectly by another way, because you have a research grant that they funded.
DG: Correct. If you look at the conflicts of interest of most oncologists who are presenting at these big conferences, almost all of them have pharmaceutical funding. You need pharmaceutical funding to continue the work, but the question is the balance. And there’s another issue: patient support groups.
GR: Patient support groups?
DG: Yes, groups of patients that try to influence decisions and regulations in ways that are supposed to benefit patients. Many patient support groups are funded by pharmaceutical companies, and there’s been a surprising lack of vocal objection to high drug prices from patient support groups because they’re essentially funded by the pharmaceutical companies.
GR: In April, we interviewed Wendell Potter, a former health insurance industry executive, who described a similar phenomenon, called “astroturfing.” Are you aware of similar cases of apparent capture?
DG: Yes. Astroturf lobbying definitely exists. This is when an organization is made to look like a grassroots organization but in fact it is artificial—without grassroots. The executive director of the Community Oncology Alliance (COA), which objected to the CMS experiment, is Ted Okon. He has very close ties to pharmaceutical companies. You could argue that he is partially lobbying on behalf of pharma. These lobbyists are able to affect legislation on many different issues.
GR: You have published a considerable number of articles on these issues. Have you ever faced pushback?
DG: Yes, nonstop.
GR: From whom? The publications themselves?
DG: Sometimes from the publications themselves, generally not. Sometimes from reviewers funded by pharmaceutical companies.
GR: What do they say? They have something to say about the science behind it?
DG: I can just say that some people don’t like the fact that I’m publishing negative things because they’re funded by pharmaceutical companies.
GR: But eventually, many of your papers were published, so they were overridden.
DG: Yes. Some editors still didn’t want it.
GR: They rejected it? Flat-out rejected it?
DG: Yes, but you can argue that’s just because of quality—I don’t want to be a bad loser!
GR: Did you publish papers anyway, even after rejection?
DG: Oh, yes. I publish in other top journals. There are many different journals, for example in the Journal of Clinical Oncology, one of the top journals in oncology. But yes, I get pushback all the time. Also from patient support groups, which are funded by pharmaceutical companies.
GR: Did you get any pushback from the U.S. hospitals where you were employed?
DG: Some.
GR: Who? What did they say?
DG: Actually, they were quite supportive of me, but certain individuals are not supportive of my work. It’s okay not to agree with the methodology of studies, or have intelligent academic discussions, but sometimes I get the feeling that pushback may be more visceral or emotional, less intellectual.
GR: How did they explain their objections?
DG: They don’t philosophically agree with my arguments.
GR: Did they mention any scientific arguments?
DG: They bring up a scientific disguise.
 GR: Didn’t you have the feeling that your job was at stake?
GR: Didn’t you have the feeling that your job was at stake?
DG: Yes. Every day I have that fear. Every day anywhere I work.
GR: How do you get this feeling? Outside people spoke to you? People looked at you in a certain way? You got disinvited?
DG: No. No, I think I’ve been welcomed positively, but there is a certain amount of refrain. My work is controversial; I like to think it is bold. Not everyone wants to be too closely aligned with me, as it could affect their own reputation—and even funding.